VUNO Secures FDA 510(k) Clearance for VUNO Med®-DeepBrain®
- Written by PR Newswire
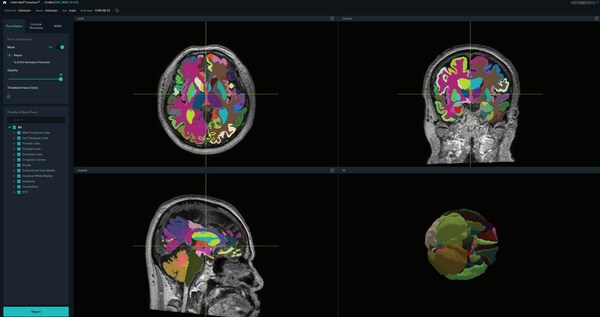
SEOUL, South Korea, Oct. 23, 2023 /PRNewswire/ -- VUNO Inc., South Korean medical AI company has received 510(k) clearance from the Food and Drug Administration (FDA) for its AI-powered brain quantification device, VUNO Med®-DeepBrain®.
VUNO Med®-DeepBrain® is intended to automate the current manual...
Read more: VUNO Secures FDA 510(k) Clearance for VUNO Med®-DeepBrain®